Drug Development & Delivery - 09/2019
SPECIAL FEATURE – Injection Devices: Wearable, Connectivity & Patient-Centric Designs Empower Self-Administration
The global self-injection devices market is expanding at a rapid pace due to high prevalence and incidence rate of chronic diseases, technological advancements, new product development and commercialization, and product differentiation strategies adopted by leading pharmaceutical companies worldwide. In terms of revenue, the global market was valued at $3.7 billion in 2017 and is projected to reach $11.3 billion by 2026.1
“The high numbers of new injectable drugs projected to reach the market in the coming years, as well as the trend to move therapies from clinics into home settings to save costs and provide more convenience for patients means increasing demands for injection devices,” says Hans Jensen, Global Business Development Director, Consort Medical, Bespak Drug Delivery Devices.
The global self-injection devices market is also driven by a significant rise in demand for home health care, owing to low cost of treatment and improvements in overall patient experience. The ability of self-administration is a key factor fueling demand for pen injectors. The pen injectors segment held a significant share of 67.6% of the market in 2017 and research indicates that it is likely to be the leading product segment, owing to the applications in diabetes, easy availability, and low cost, according to a report from Transparency Market Research.
Technological advancements in self-injection devices, especially in autoinjectors and wearable injectors, for the administration of high-viscosity and large-volume drugs represents a potential business development opportunity for leading players. Reports suggest that the wearable injectors segment is projected to expand at a CAGR of 20% between 2018 and 2026.1
“Traditional spring-based autoinjectors have previously been sufficient to deliver the drugs being developed, however many biologics by nature need higher doses to have an effect, which leads to higher viscosities and/or higher volumes to be delivered,” says Mr. Jensen. “Furthermore, others are working on viscous long-acting formulations to decrease frequency of injections, thereby increasing patient convenience. Finally, a number of drugs initially developed for IV administration in clinics are being re-formulated to allow home administration, but that also can result in high viscosity and/or higher doses that may not be appropriate for traditional autoinjector devices.”
This exclusive Drug Development & Delivery report highlights the innovation in injection devices – from wearables to connectivity to varied dose administration – that have occurred in the past year.
Kahle Automation: Micro-Assemblies & Components for Wearable Injection Devices
As products are getting smaller and tolerances are getting tighter, this puts pressure on the automation suppliers and component suppliers to produce high-volume components at much tighter tolerances. These micro-assemblies and associated micro-components are crucial to the functionality of today’s complex drug delivery and diagnostic devices, states Julie Logothetis, President of Kahle Automation.
Kahle designs and builds automation equipment for micro-assemblies with production outputs ranging from 10ppm to 630ppm. Its knowledge includes the feeding and assembly of molded components, tubing, and cannula as small as 6mm long and 32G. Its equipment can integrate micro-dispensing, vision and leak testing, punching filters and diagnostic mediums, welding, and cannula bending.
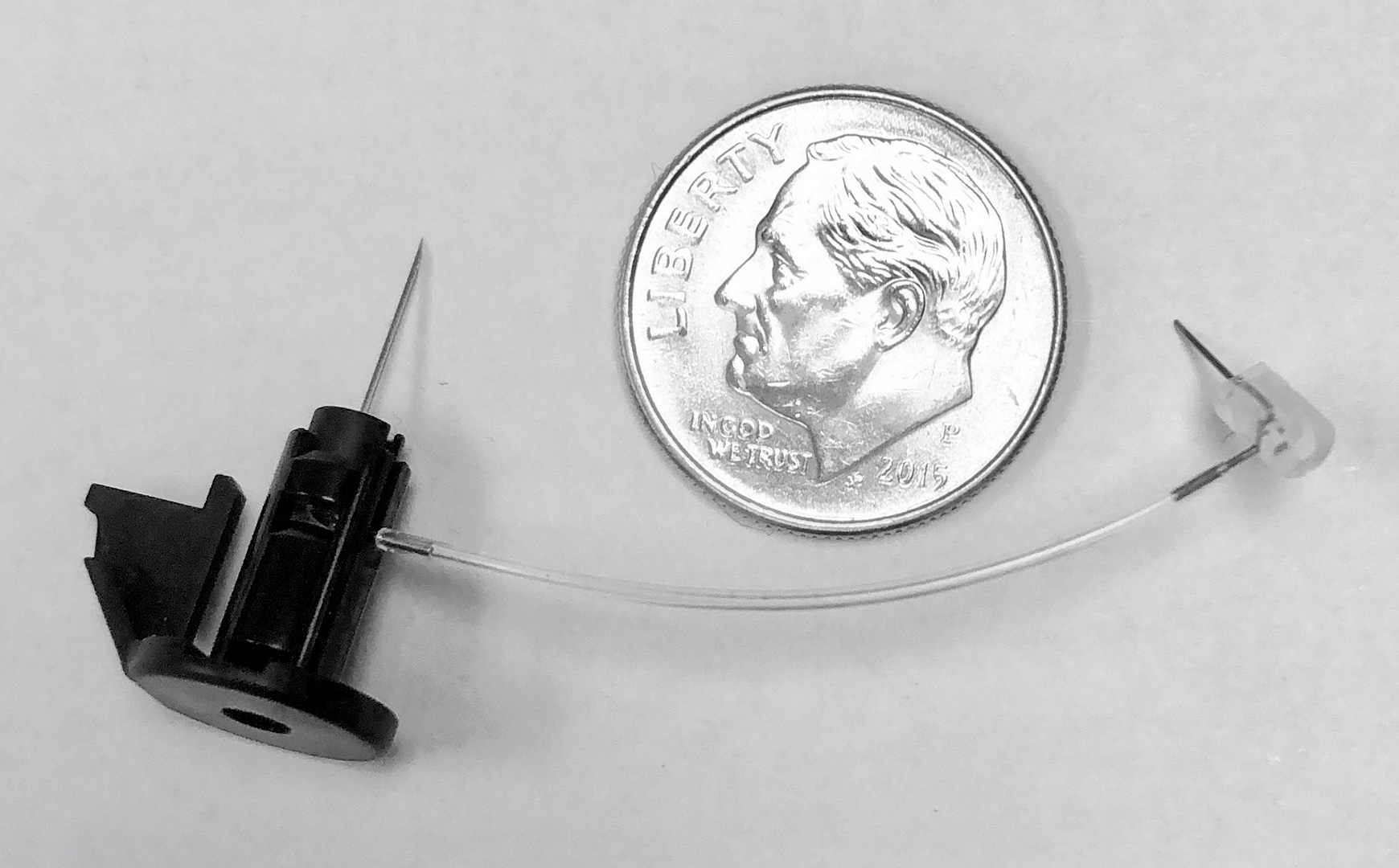
Micro Fluid Path Assembly by a Kahle Machine.
Kahle recently completed several projects involving the assembly of the internal fluid path of wearable injection devices. “It is critical for these products to keep the fluid path patent even through the complex maze of tubing and bent cannula required to navigate the path within the internal working of the device,” says Ms. Logothetis. Kahle offers cannula bending technology that can bend 29G cannula up to 120 degrees while maintaining orientation of the ground sharp.
In addition, Kahle has provided the automation for the manufacture of an ocular injection device that will allow the practitioner to perform a precision injection in the eye to the exact point of treatment, she says.
Article sourced from www.drug-dev.com SPECIAL FEATURE – Injection Devices: Wearables, Connectivity & Patient-Centric Designs Empower Self-Administration
